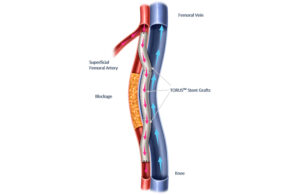
Detour features the EndoCross device and Torus stent grafts. It enables physicians to bypass lesions in the superficial femoral artery by using stents routed through the femoral vein. This restores blood flow to the leg, effectively treating patients with long lesions (20cm-46cm in length), Endologix says. It also treats those who already faced failed endovascular procedures or those who represent suboptimal open surgical bypass candidates.
The FDA approved Detour for use in percutaneous transmural arterial bypass (PTAB) to treat PAD in May 2023.
Endologix said three-year DETOUR2 data highlighted the durable efficacy of the Detour system, comparable to open bypass with a synthetic graft. Investigators reported low rates of complications and deep venous thrombosis (DVT), demonstrating its favorable safety profile.
DETOUR2 enrolled 202 patients across 32 sites, with 200 total patients treated with the Detour system. The company reported a mean lesion length of 32.7 cm, with 96% chronic total occlusions (CTO), and 70% severely calcified.
Freedom from CD-TLR (clinically driven target lesion revascularization) came in at 66.8%, with primary patency at 58.2%. Endologix recorded 96.7% clinical success and 95.9% freedom from symptomatic DVT. The company also saw freedom from major limb amputation of 98.5% and an average hospital stay length of 1.1 days.
“The 36-month data from the DETOUR2 Study underscores the potential of the DETOUR System to significantly impact the treatment paradigm for long segment SFA disease,” said Dr. Matt Thompson Endologix president, and CEO. “We are confident in the DETOUR system’s ability to offer a less invasive, effective alternative for patients with challenging femoropopliteal lesions.”

