Under the agreement, Cook Medical will assume commercial responsibilities for this Bentley product.
“We are excited to welcome Bentley’s BeBack catheter to our peripheral intervention portfolio,” Alec Cerchiari, director of product management for Cook’s PAD and Venous specialty, said in a news release. “The BeBack catheter is a unique tool for crossing heavily calcified lesions in antegrade or retrograde fashion. It complements Cook’s robust portfolio of above- and below-the-knee products, including our Micropuncture Pedal Introducer Access Sets, Flexor guiding sheaths and CXI support catheter.”
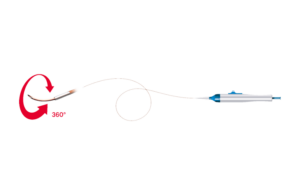
BeBack is a crossing catheter that Bentley designed to steer through chronic total occlusions (CTO) and provide targeted re-entry options. The catheter features a steerable and adjustable nitinol needle, a radiopaque marker on the tip to indicate the direction of needle curves, and 80 and 120cm lengths in 2.9Fr and 4Fr sizes.
“The BeBack catheter is an effective approach for successful recanalisation and should be available in every lab across the U.S. Thanks to this collaboration with Cook, we will now have a better commercial footprint in the U.S. with even more opportunities to treat patients with debilitating arterial and venous disease,” Bentley Director of Sales and Marketing Martijn Nugteren said.

