Confluent Medical Technologies announced today that it now offers REACH and EU MDR-compliant polyimide tubing. REACH, a European Union regulation, imposes stringent limitations for the EU market. It covers the amount of restricted substances contained in devices sold in the EU. It stands for Registration, Evaluation, Authorization, and … [Read more...] about Confluent Medical now offers EU MDR-compliant polyimide tubing
Tubing Components
FDA clears Gravitas Medical’s smart feeding tube
Gravitas Medical this week announced it received FDA 510(k) clearance for its Enteric feeding tube system and feeding tube. The Enteral nutrition delivery system has a "smart" nasogastric tube that is equipped with sensors to provide information about the location of the tube tip relative to the stomach and monitor. Together, the sensors and the … [Read more...] about FDA clears Gravitas Medical’s smart feeding tube
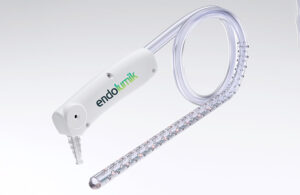
Endolumik’s illuminated device takes a big step for safety
Endolumik's story started a few years ago as the FDA sounded the alarm over the risks of internal surgical staplers. In 2019, the federal agency warned healthcare providers that it had received more than 41,000 medical device reports (MDRs) related to surgical staplers and staples for internal use from 2011 to 2018. Those MDRs tallied more than … [Read more...] about Endolumik’s illuminated device takes a big step for safety
FDA clears first device authorized under Safer Technologies Program
The FDA last week cleared a new gastric calibration tube, marking the first authorization of a device under the agency's Safer Technologies Program. FDA launched its Safer Technologies Program in 2021. It modeled the program on its breakthrough devices program. The Safer Technologies Program (STeP) covers devices that could improve the safety of … [Read more...] about FDA clears first device authorized under Safer Technologies Program
How Penumbra’s smart-sucking algorithms and catheters speed up clot removal
Blood loss is a big problem when using aspiration catheters to remove blood clots from patients' veins and arteries. "You suck out the clot, you also suck out the blood," said Sandra Lesenfants, president of the interventional business at Penumbra (NYSE:PEN). Algorithms developed by Penumbra's software team for the company's continuous … [Read more...] about How Penumbra’s smart-sucking algorithms and catheters speed up clot removal
Freudenberg Medical expands medical tubing production to Massachusetts
NEWS RELEASE: Freudenberg Medical Expands Medical Tubing Production to Massachusetts Freudenberg Medical, a global contract design and manufacturing provider to the medical device and pharmaceutical industry, has added custom silicone extrusion and medical and biopharma tube processing to its newest cleanroom manufacturing operation and global … [Read more...] about Freudenberg Medical expands medical tubing production to Massachusetts
Cirtec Medical acquires QMD Precision Components
Cirtec Medical (Brooklyn Park, Minnesota) announced today that it has acquired QMD's Precision Components business. The financial terms of the deal were not disclosed. The move comes amid continued consolidation in the medical device industry's contract manufacturing and supplier space. QMD’s Precision Components business specializes in the … [Read more...] about Cirtec Medical acquires QMD Precision Components
Why Affera’s cardiac ablation technology is worth $1B to Medtronic
Affera started in 2014 with a simple goal that paid off when Medtronic (NYSE:MDT) bought the company for up to $1 billion this year. Achieving that goal, however, took some unconventional and sometimes difficult design choices, Affera founder and CEO Doron Harlev said. Newton, Massachusetts–based Affera's system diagnoses, maps and treats … [Read more...] about Why Affera’s cardiac ablation technology is worth $1B to Medtronic
NuVasive launches Tube System and Excavation Micro for posterior spine surgery
NuVasive this week announced the commercial launch of its NuVasive Tube System (NTS) and Excavation Micro for minimally invasive posterior spine surgery. The San Diego-based orthopedic company designed the NTS and Excavation Micro to provide comprehensive solutions for TLIF and decompression procedures. It adds to the company's access and … [Read more...] about NuVasive launches Tube System and Excavation Micro for posterior spine surgery
Freudenberg Medical introduces HelixFlex TPE tubing
Freudenberg Medical this week announced the launch of its HelixFlex high-purity thermoplastic elastomer tubing. The company says it designed the HelixFlex tubing for biopharmaceutical fluid transfer applications. The expanded offering adds to Freudenberg's existing pharma product portfolio of silicone tubing and components for bioprocessing, … [Read more...] about Freudenberg Medical introduces HelixFlex TPE tubing