Vantis Vascular announced today that it received FDA 510(k) clearance for its CrossFast integrated microcatheter guide extension system. San Jose, California-based Vantis develops the CrossFast system along with the CrossShock intravascular lithotripsy (IVL) system. It designed CrossFast to help physicians perform faster, easier and safer … [Read more...] about Vantis Vascular wins FDA nod for integrated microcatheter guide extension system
Catheters
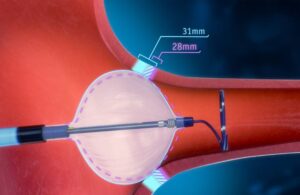
Jupiter Endovascular reports first patient treated in U.S. study of Vertex pulmonary embolectomy system
Jupiter Endovascular today announced the first U.S. patient treated in its SPIRARE II U.S. pivotal study for the Vertex system. The first U.S. patient treated follows last month's announcement of the first Vertex use in the SPIRARE I study in Poland. SPIRARE II evaluates the Vertex pulmonary embolectomy system in the treatment of acute pulmonary … [Read more...] about Jupiter Endovascular reports first patient treated in U.S. study of Vertex pulmonary embolectomy system
FDA approves IDE for Medtronic Prevail drug-coated balloon
Medtronic (NYSE: MDT) announced today that it received FDA investigational device exemption (IDE) for its Prevail drug-coated balloon (DCB). The medtech giant can now begin a pivotal clinical trial for the coronary paclitaxel DCB for in-stent restenosis (ISR) and de novo small vessel disease. It plans to use data from its Prevail Global Clinical … [Read more...] about FDA approves IDE for Medtronic Prevail drug-coated balloon
Nerve ablation tech developer Autonomix to effect a reverse stock split
Autonomix (Nasdaq:AMIX) announced today that it filed to effect a 1-for-20 reverse stock split of its common stock. The Woodlands, Texas-based Autonomix plans for the split to take effect at 11:59 p.m. ET on Oct. 24, 2024. It plans for its common stock to open trading on the Nasdaq market on Oct. 25, 2024, on a post-split basis, under the … [Read more...] about Nerve ablation tech developer Autonomix to effect a reverse stock split
Peytant wins FDA de novo nod for covered stent system
Peytant Solutions announced today that the FDA granted marketing authorization for its AMStent tracheobronchial covered stent system. Plymouth, Minnesota-based Peytant designed its AMStent to treat pulmonary obstructions caused by cancer. The novel, proprietary therapy platform is indicated for treating tracheobronchial strictures produced by … [Read more...] about Peytant wins FDA de novo nod for covered stent system
Gradient adds former Edwards exec to board as FDA approves trial expansion
Gradient Denervation Technologies announced today that it appointed medical device entrepreneur and executive Stanton Rowe to its board. Paris-based Gradient also said the FDA approved an expansion of its early feasibility study of its hypertension treatment. The company develops a minimally invasive, ultrasound-based catheter device for … [Read more...] about Gradient adds former Edwards exec to board as FDA approves trial expansion
Okami Medical closes $32.5M financing to support catheter tech for peripheral vascular occlusion
Okami Medical announced today that it closed a $32.5 million financing round to support its catheter-based technologies. Aliso Viejo, California-based Okami develops multiple technologies for peripheral vascular occlusion. To date, it developed the LOBO (low-profile braided occluder) vascular occlusion system and the Sendero … [Read more...] about Okami Medical closes $32.5M financing to support catheter tech for peripheral vascular occlusion
Johnson & Johnson reports first commercial Varipulse PFA use in Canada
Johnson & Johnson MedTech [WtwhTicker symbol="JNJ"](NYSE: JNJ)[/WtwhTicker] today announced the first completed commercial case with its Varipulse platform in Canada. The company said in a post on LinkedIn that doctors at the Ottawa Heart Institute completed the first commercial case with Varipulse. Johnson & Johnson said the procedure … [Read more...] about Johnson & Johnson reports first commercial Varipulse PFA use in Canada
Boston Scientific warns on some PolarX cryoablation catheters following reports of death
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] issued an urgent field safety notice in Europe to notify customers of updates to its PolarX cryoablation catheters. Marlborough, Massachusetts-based Boston Scientific's notice relates to the PolarX and PolarX FIT cryoablation catheters. These systems work as part of the PolarX … [Read more...] about Boston Scientific warns on some PolarX cryoablation catheters following reports of death
Amplitude Vascular enrolls first patient in IVL study
Amplitude Vascular Systems (AVS) announced today that it enrolled the first patient(s) in the U.S. pivotal trial for its intravascular lithotripsy (IVL) technology. Boston-based Amplitude Vascular's POWER PAD II clinical study evaluates the safety of the Pulse IVL system. The company's pulsatile IVL (PIVL) therapy treats patients with moderate … [Read more...] about Amplitude Vascular enrolls first patient in IVL study