Supira Medical announced today that it began patient enrollment in a U.S. early feasibility trial of its percutaneous ventricular assist device (pVAD). The Shifamed portfolio company plans to evaluate the pVAD in patients undergoing high-risk percutaneous coronary intervention (HRPCI). Los Gatos, California-based Supira’s study looks at the … [Read more...] about Supira begins early feasability study for percutaneous ventricular assist device
Technologies & Devices
Medtronic acquires articulating instrument maker Fortimedix Surgical
Fortimedix Surgical announced today that it has been acquired by medtech giant Medtronic (NYSE:MDT). "We are pleased to share that Fortimedix Surgical was acquired by Medtronic," the company wrote on LinkedIn. "A unique opportunity to leverage our articulating instrument technology for application across patient therapies. We look forward to … [Read more...] about Medtronic acquires articulating instrument maker Fortimedix Surgical
Route 92 Medical picks up CE mark for stroke portfolio
Route 92 Medical announced today that it received CE mark for its line of neurovascular intervention products for treating stroke. The company also picked up clearance under the MDSAP program, giving broad access to international markets. Authorization covers the majority of the company's products, including: HiPoint 70/Tenzing 7 … [Read more...] about Route 92 Medical picks up CE mark for stroke portfolio
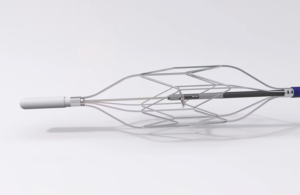
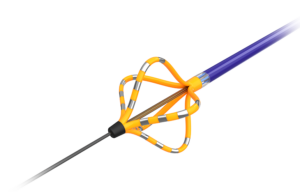
EndoTheia completes first-in-human study of steerable kidney stone basket
EndoTheia announced today that it completed the first-in-human clinical trial for its FlexStone basket, a steerable kidney stone treatment. Nashville, Tennessee-based EndoTheia said its FlexStone basket represents the first-ever independently steerable kidney stone basket. It enables precision and control in kidney stone removal by letting … [Read more...] about EndoTheia completes first-in-human study of steerable kidney stone basket
Second Heart Assist’s Whisper device has successful case studies
Second Heart Assist (Salt Lake City) today announced successful case studies in South America with its Whisper catheter-delivered heart assist device. Dr. Adrian Ebner, head of the cardiovascular department at the Sanatorio Italiano Hospital in Asuncion, Paraguay, and his team completed non-randomized case studies involving six patients. Second … [Read more...] about Second Heart Assist’s Whisper device has successful case studies
JenaValve to begin Trilogy heart valve trial
JenaValve announced today that it received the green light to begin a new randomized controlled trial for its Trilogy device. Irvine, California-based JenaValve can now start the ARTIST trial, slated for early next year. The company — set to be acquired by Edwards as part of a $1.2 billion double M&A swoop alongside Endotronix — also … [Read more...] about JenaValve to begin Trilogy heart valve trial
Boston Scientific resumes study of pulse field ablation as first-line AFib therapy
Boston Scientific (NYSE: BSX) is resuming its “Avant Guard” study of pulse field ablation as a first-line therapy for persistent atrial fibrillation (AFib). The device developer paused the trial last month, with CEO Mike Mahoney citing the need to “assess a few unanticipated observations.” Boston Scientific said it did not halt the trial due to … [Read more...] about Boston Scientific resumes study of pulse field ablation as first-line AFib therapy
Johnson & Johnson MedTech wins FDA nod for Varipulse PFA
Johnson & Johnson MedTech announced today that the FDA approved its Varipulse pulsed field ablation (PFA) platform for treating AFib. Varipulse treats AFib with a single device that combines PFA with the Carto 3 mapping system. With approval, Johnson & Johnson joins Medtronic and Boston Scientific as companies with PFA technologies … [Read more...] about Johnson & Johnson MedTech wins FDA nod for Varipulse PFA
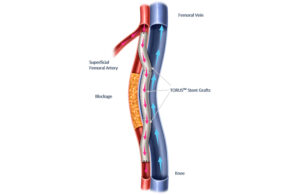
Endologix reports positive 3-year Detour PAD treatment data
Endologix announced the final 36-month results for the DETOUR2 study evaluating its Detour system for treating peripheral arterial disease (PAD). Detour features the EndoCross device and Torus stent grafts. It enables physicians to bypass lesions in the superficial femoral artery by using stents routed through the femoral vein. This restores … [Read more...] about Endologix reports positive 3-year Detour PAD treatment data
Penumbra has positive data for computer-assisted vacuum thrombectomy system
Penumbra (NYSE:PEN) today announced new data demonstrating improved outcomes with its computer-assisted vacuum thrombectomy (CAVT) technology. Data evaluated the use of the CAVT technology in people with intermediate-risk pulmonary embolism (PE). Results showed that those treated with CAVT had shorter hospital stay lengths, shorter … [Read more...] about Penumbra has positive data for computer-assisted vacuum thrombectomy system