Solaris Endovascular today announced its formation to develop solutions for dialysis access and treating peripheral artery disease (PAD). The New Orleans-based company develops technologies to treat and extend the patency of dialysis access fistulae, grafts, and peripheral arteries. It aims to enable patients to lead healthier lives with fewer … [Read more...] about Vascular stent graft maker Solaris Endovascular launches to address vascular health challenges
Stents
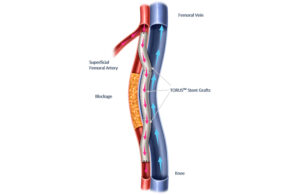
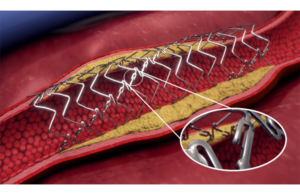
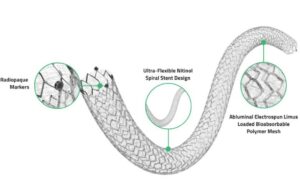
Endologix reports positive 3-year Detour PAD treatment data
Endologix announced the final 36-month results for the DETOUR2 study evaluating its Detour system for treating peripheral arterial disease (PAD). Detour features the EndoCross device and Torus stent grafts. It enables physicians to bypass lesions in the superficial femoral artery by using stents routed through the femoral vein. This restores … [Read more...] about Endologix reports positive 3-year Detour PAD treatment data
Abbott reports sustained benefits with Esprit drug-eluting stent
Abbott (NYSE:ABT) today announced data demonstrating the long-term effectiveness of its Esprit BTK drug-eluting stent. The everolimus-eluting resorbable scaffold system treats chronic limb-threatening ischemia (CLTI) below the knee (BTK). Abbott designed it to keep arteries open and deliver everolimus to support vessel healing prior to … [Read more...] about Abbott reports sustained benefits with Esprit drug-eluting stent
R3 Vascular wins FDA IDE for drug-eluting scaffold
R3 Vascular announced today that the FDA granted investigational device exemption (IDE) to evaluate its Magnitude drug-eluting bioresorbable scaffold. Mountain View, California-based R3 Vascular designed Magnitude for treating below-the-knee (BTK) peripheral arterial disease (PAD). With the FDA granting IDE, it can now initiate the ELITE-BTK … [Read more...] about R3 Vascular wins FDA IDE for drug-eluting scaffold
Elixir Medical has positive data for drug-eluting bioadaptive implant
Elixir Medical announced positive data demonstrating the benefit of its DynamX coronary bioadaptor system in target lesion failure (TLF). Data highlighted the success of DynamX compared to Medtronic's Resolute Onyx zotarolimus drug-eluting stent (DES). The study looked at complex patient populations within the INFINITY-SWEDEHEART randomized … [Read more...] about Elixir Medical has positive data for drug-eluting bioadaptive implant
Peytant wins FDA de novo nod for covered stent system
Peytant Solutions announced today that the FDA granted marketing authorization for its AMStent tracheobronchial covered stent system. Plymouth, Minnesota-based Peytant designed its AMStent to treat pulmonary obstructions caused by cancer. The novel, proprietary therapy platform is indicated for treating tracheobronchial strictures produced by … [Read more...] about Peytant wins FDA de novo nod for covered stent system
Medinol has first human implant of drug-eluting peripheral stent in Australia
Medinol today announced the successful first-in-human implantation of its ChampioNIR drug-eluting peripheral stent. Dr. Gerard S. Goh and Dr. Thodur Vasudevan of the Alfred Hospital in Melbourne, Australia, completed the stent implant. The company says it introduces a "revolutionary advancement" in the mechanics, durability and drug delivery of … [Read more...] about Medinol has first human implant of drug-eluting peripheral stent in Australia
InspireMD wins FDA IDE for carotid stent study
InspireMD (Nasdaq:NSPR) announced today that the FDA granted investigational device exemption (IDE) for its CGuard Prime 80cm carotid stent system. The FDA says the company can begin the CGUARDIANS II pivotal study of the system used during transcarotid revascularization (TCAR) procedures. InspireMD picked Dr. Patrick Geraghty and Dr. Patrick … [Read more...] about InspireMD wins FDA IDE for carotid stent study
Edwards launches Sapien 3 valve with Alterra prestent in Europe
Edwards Lifesciences (NYSE:EW) announced that it launched its Sapien 3 transcatheter pulmonary valve implant (TPVI) system with Alterra adaptive prestent in Europe. The launch expands the continent’s minimally invasive treatment options to a broader range of patients with congenital heart conditions. Edwards recently received CE mark for … [Read more...] about Edwards launches Sapien 3 valve with Alterra prestent in Europe
InspireMD submits carotid stent for FDA premarket approval
InspireMD (Nasdaq:NSPR) announced today that it submitted a premarket approval (PMA) application to the FDA for its CGuard system. The company designed the CGuard Prime carotid stent system, made from nitinol, for the treatment of carotid artery stenosis. It based its PMA application on positive one-year data from the company's C-GUARDIANS … [Read more...] about InspireMD submits carotid stent for FDA premarket approval