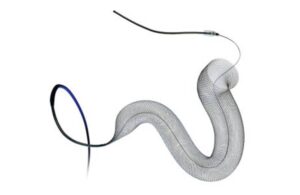
Q’Apel Medical announced that the FDA classified a voluntary recall of its 072 Aspiration System as Class I, the most serious kind. The company earlier this year initiated a discontinuation and recall of the system, also known as "Hippo," which includes "Cheetah." It recalled 1,617 units of the Hippo system after receiving an FDA warning letter. … [Read more...] about FDA says Q’Apel aspiration system recall is Class I following discontinuation
Recalls
BD warns on intravascular catheter
The FDA shared an alert that BD (NYSE: BDX) and its Bard subsidiary warned customers of an issue with certain intravascular catheters. BD recommended the removal of certain unused PowerPICC intravascular catheters from use or sale. In-use PowerPICC intravascular catheters also received updated instructions for use. Affected products include the … [Read more...] about BD warns on intravascular catheter
Conavi warns on intravascular catheter issue
The FDA today issued an alert related to a potentially high-risk device issue with certain Conavi Novasight Hybrid catheters. Its communication comes as part of its pilot to enhance the medical device recall program. The FDA said it became aware of the issue after Conavi sent a letter to affected customers recommending the removal of certain … [Read more...] about Conavi warns on intravascular catheter issue
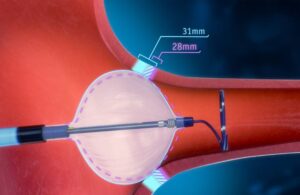
Medtronic recalls embolization device after reported deaths
The FDA deemed a recall of some Medtronic Pipeline Vantage embolization devices serious after multiple deaths related to the device. The recall involves removing Pipeline Vantage 027 device models from use and sale and updating instructions for Pipeline Vantage 021 models. Medtronic's Pipeline Vantage embolization devices with Shield Technology … [Read more...] about Medtronic recalls embolization device after reported deaths
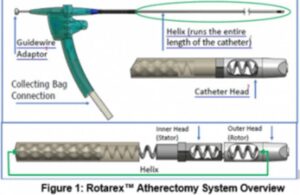
BD warns on atherectomy catheters after deaths
BD (NYSE: BDX) recently issued a medical device correction later related to its Bard subsidiary's Rotarex atherectomy system. The company identified a number of anatomical factors that could contribute to catheter helix fracture and/or breakage. It reports 30 serious injuries and four deaths associated with the issue. Additionally, BD reported … [Read more...] about BD warns on atherectomy catheters after deaths
Medline has a Class I arterial catheter recall
The FDA labeled a recall of integrated arterial catheters made by Medline Class I, the most serious kind of recall. This recall involves removing certain devices from where they are used or sold. The company reports no injuries or deaths related to this recall to date, according to an FDA notice. Medline designed its arterial catheter for … [Read more...] about Medline has a Class I arterial catheter recall
Boston Scientific updates instructions for cryoablation catheters after reported deaths
The FDA issued a notice alerting customers to updates made by Boston Scientific to its PolarX cryoablation catheters. This recall involves updating instructions for use (IFU), not removing devices from the market. The FDA identified it as the most serious type of recall, though, as it may cause serious injury or death. Boston Scientific … [Read more...] about Boston Scientific updates instructions for cryoablation catheters after reported deaths
Boston Scientific warns on some PolarX cryoablation catheters following reports of death
Boston Scientific [WtwhTicker symbol="BSX"](NYSE: BSX)[/WtwhTicker] issued an urgent field safety notice in Europe to notify customers of updates to its PolarX cryoablation catheters. Marlborough, Massachusetts-based Boston Scientific's notice relates to the PolarX and PolarX FIT cryoablation catheters. These systems work as part of the PolarX … [Read more...] about Boston Scientific warns on some PolarX cryoablation catheters following reports of death
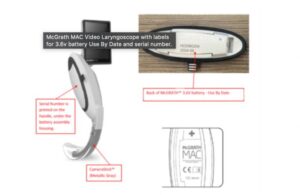
Medtronic recalls McGrath Mac laryngoscope due to potential for explosions
The FDA issued a notice deeming a recall of Medtronic [WtwhTicker symbol="MDT"](NYSE: MDT)[/WtwhTicker] McGrath Mac video laryngoscopes Class I, the most serious kind. This recall involves removing certain devices from where they are used or sold as the device may cause serious injury or death if people continue using it. It also includes … [Read more...] about Medtronic recalls McGrath Mac laryngoscope due to potential for explosions
Medtronic endotracheal tubes recall sparks an FDA warning
The FDA issued a notice instructing healthcare providers to stop using certain reinforced endotracheal tubes made by Medtronic (NYSE:MDT). This warning extends to NIM Contact EMG reinforced endotracheal tubes and NIM (Standard) EMG reinforced endotracheal tubes. Medtronic offers the neural integrity monitor (NIM) electromyogram (EMG) tubes for … [Read more...] about Medtronic endotracheal tubes recall sparks an FDA warning