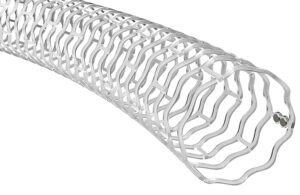
NEWS RELEASE: Confluent Medical Technologies announces Ultra Polyimide Confluent unveils material breakthrough that delivers twice the strength of traditional polyimide. Scottsdale, Arizona – Confluent Medical Technologies (Confluent), a leading medical device and materials science contract manufacturer specializing in polymer and metal … [Read more...] about Confluent Medical Technologies announces Ultra Polyimide
Plastics
Zeus to launch next-gen film-cast PTFE catheter liners
NEWS RELEASE: Zeus to launch next-gen film-cast PTFE liners engineered for flexibility and superior strength Unveiling at MD&M West, the new StreamLiner NG catheter liners minimize defects and maximize performance versus existing film-cast PTFE liners. Orangeburg, S.C. – Zeus, a global leader in advanced polymer solutions and catheter … [Read more...] about Zeus to launch next-gen film-cast PTFE catheter liners
Bioabsorbable polymers for implantable medical devices: What to know
Bioabsorbable polymers are used for an expanding range of medical devices.
Bioabsorbable polymers degrade and disappear at predictable rates, making them an ideal material for parts of implantable devices that could otherwise impair healing or create an ongoing risk of injury or infection.
Bioabsorbable sutures made of glycolide/lactide polymers, first developed in the 1970s, are strong and flexible enough to hold … [Read more...] about Bioabsorbable polymers for implantable medical devices: What to know
This medtech developer is throwing everything at clots
Retriever Medical is developing a thrombectomy system with a four-front approach for treating blood clots. The Las Vegas-based startup calls its technology VORS AMD, for vascular occlusion removal system with aspiration, mechanical and drug delivery. The approach combines nitinol baskets for mechanical thrombectomy with aspiration, clot-busting … [Read more...] about This medtech developer is throwing everything at clots
The 24 best medical device innovations of 2022
The Galien Foundation recently announced its nominees of medical device innovations for its 2022 Prix Galien USA awards. There are 24 medical technologies nominated for the annual award this year, up from 18 nominees in 2021. The Galien Foundation’s annual Prix Galien awards highlight devices, biotechnology and pharmaceutical products … [Read more...] about The 24 best medical device innovations of 2022
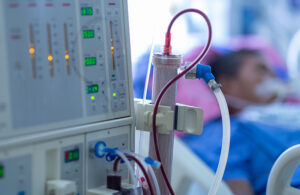
FDA warns of potential toxic risk from Fresenius hemodialysis machines
The FDA is evaluating the risk of exposure to toxic chemicals from silicone tubing used in Fresenius Medical Care (NYSE: FMS) hemodialysis machines. The investigation concerns three models of Fresenius hemodialysis machines: the 2008T, 2008K2, and 2008K. The chemicals — non-dioxin-like (NDL) polychlorinated biphenyl acids (PCBAs) and NDL … [Read more...] about FDA warns of potential toxic risk from Fresenius hemodialysis machines
FDA warns of risk from Medtronic silicone-based EMG endotracheal tubes
The FDA is evaluating the risk of Medtronic (NYSE:MDT) silicone-based electromyogram (EMG) endotracheal tubes following reports of deaths and serious adverse events. The FDA issued the warning last week in a letter to healthcare providers concerning the Medtronic NIM Standard Reinforced EMG Endotracheal Tubes and Medtronic NIM Contact Reinforced … [Read more...] about FDA warns of risk from Medtronic silicone-based EMG endotracheal tubes
Plastics and medical devices: Changes for safety and cost
The move toward polypropylene and polyethylene in medical devices requires new manufacturing and assembly solutions such as ultrasonic and laser welding. Didier Perret, Emerson Plastics are ubiquitous in medical applications thanks to their light weight, durability and flexibility, among other attributes. However, concern has been increasing … [Read more...] about Plastics and medical devices: Changes for safety and cost
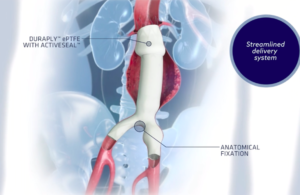
FDA committee to examine safety of endovascular stent grafts
FDA’s CDRH Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet later this year to examine the safety of endovascular stent grafts made by Endologix and other companies. Day one of the meeting will examine the benefit-risk profile of the Endologix AFX endovascular graft system regarding a potentially … [Read more...] about FDA committee to examine safety of endovascular stent grafts
Freudenberg Medical opens new HQ in larger Mass. factory
Freudenberg Medical announced today that it has completed construction of a new medical manufacturing operation in Beverly, Mass. The new building will serve as Freudenberg Medical's global headquarters and will manufacture medical devices and components in medical and implant-grade silicones and high-performance plastics. The 36,000 ft2 … [Read more...] about Freudenberg Medical opens new HQ in larger Mass. factory