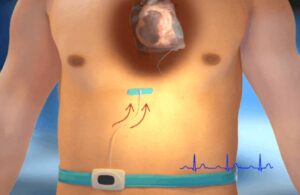
The study evaluates PercAssist’s minimally invasive extravascular platform that provides hemodynamic support for chronic heart failure patients.
Principal investigator Dr. Petr Neuzil completed the first case at the Na Homolce Hospital in Prague, Czech Republic. Also involved were co-investigators Dr. Ivo Skalsky and Dr. Marek Janotka. Neuzil and his team successfully deployed the PercAssist percutaneous synchronized cardiac assist system (PSCA).
The PSCA system features a balloon-based catheter and console. It inflates and deflates in synchrony with the patient’s cardiac rhythm to provide hemodynamic support. Santa Clara, California-based PercAssist said its system was successfully deployed in the study. It provided hemodynamic stability immediately following implantation and throughout the implant period, the company said.
“This extravascular ventricular assist technology has tremendous potential for providing hemodynamic support for heart failure patients without the need for anticoagulation therapy,” Neuzil said. “Our team is excited to evaluate this innovative technology; it is a revolutionary advance for our field.”
The system enables first-line interventional cardiologists and cardiac surgeons to provide hemodynamic support for an acute event. These include cardiogenic shock or acute decompensated heart failure.
PercAssist plans to complete its first-in-human study and follow it with a multi-center feasibility and pivotal trial under FDA investigational device exemption.
“This successful first-in-human case comes after three years of rigorous design and development activities, including design verification and validation testing, and numerous pre-clinical acute and chronic assessments,” said Gerardo Noriega, president and CEO of PercAssist. “This milestone marks the beginning of our efforts to bring this potentially groundbreaking, novel technology to heart failure patients on a global scale.”
