 AtHeart Medical is initiating a U.S. investigational device exemption trial for its atrial septal defects (ASD) occluder.
AtHeart Medical is initiating a U.S. investigational device exemption trial for its atrial septal defects (ASD) occluder.
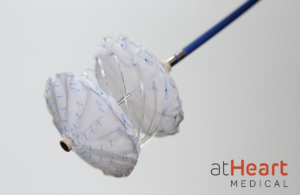
The reSept ASD Occluder is an occluder with a metal-free, bioresorbable frame. The implant is designed to overcome the limitations of occluders by reducing the risk of complications associated with the long-term presence of metal in the heart and to preserve future treatment options that require transseptal intervention.
“I am excited to lead atHeart Medical and for the potential of our first product, the reSept ASD Occluder, for patients requiring ASD closure,” CEO Laurent Grandidier said. “With such an experienced team, we have hit the ground running and are focused on preparing initial clinical sites in our U.S. IDE pivotal trial.”
AtHeart’s Ascent-ASD pivotal trial will evaluate the safety and efficacy of the reSept ASD Occluder for treating patients with clinically significant, isolated ASD. The prospective, single-arm investigation will enroll up to 250 patients at global sites.
“Currently available occluders leave a significant amount of metal in the heart, which could cause issues later in life,” Larry Latson, co-principal investigator on the study and director of pediatric interventional cardiology at the Joe DiMaggio Children’s Hospital in Hollywood, Fla., said in a news release. “The unique design of this bioresorbable frame is promising, especially for my younger patients. I look forward to this upcoming trial.”
ASD is an opening in the septum between the left and right atria. It is often described as a “hole in the heart,” according to atHeart Medical. Most ASD cases are congenital defects and affect six in 10,000 births, but it can also be the result of a procedure that required transseptal crossing. If untreated, the right side of the heart could eventually enlarge and weaken. Blood pressure in the lungs could also increase and lead to pulmonary hypertension. Occluders implanted through a minimally invasive procedure is the standard of care for treating ASD.
